Introduction
RedOak Instruments (ROI) is a sports/medical technology company that non-invasively measures fine motor degradation to indicate the presence of a concussion or injury. Sports trainers, coaches, EMTs, and medical practitioners can monitor their rehabilitation programs with our technology and document evidence of injury for a conclusive diagnosis by the practitioners.
Deal Highlights
- FDA approved medical instrument
- Quick and Non-Invasive
- Equipment ready to sell
- MassChallenge company finalist
Recommendations

Testimonials

“Stroke rehabilitation is difficult to monitor. By testing the patient, before and after acupuncture treatments, the medical staff can document the treatment outcome with objective data. The individual has given up on recovery when he is convinced to try again by his family. Upon seeing the results his treatment is having on hand control, the patient is encouraged to continue with the treatment and is able to regain most of his control of the left hand. Improvements were also seen in the right hand, but to a lesser extent.”
The results of this treatment are presented/published in the American Public Health Association (APHA) 2017 Annual Meeting & Expo (Nov 4 – Nov 8) Abstract #380447: “Utilizing Fine Motor Control Measurements for Outcome-Based Acupuncture Practice: An Evidence-Based Research Study”
Problem
Athletes will not report injuries they feel will remove themselves from active play or admit they are not the best athlete. Untreated injuries result in diminished capabilities and poor team results. Closed head injuries, concussions, and mild traumatic brain injuries (mTBI)are difficult to diagnose due to the absence of apparent injury. The injured athlete often denies he or she is even injured. Cognitive symptoms due to the mTBI injury may not appear for weeks.

Solution
Red Oak Instrument’s medical device monitors the fine motor control capabilities of sports team members. This allows trainers, coaches, and medical staff to improve capabilities of the team while screening and treating unreported injuries. By monitoring reaction times and hand coordination, small injuries may be detected prior to becoming serious injuries as shown in the figure on sports management below. These measurements may be used as a screening test which detects and quantifies symptoms of mTBI in less than ten minutes. Identifying and documenting a physical injury, protects an injured patient from re-injury.The technology also monitors recovery and rehabilitation rates, and identifying when a patient has fully returned to normal. ROI’s Fine Motor Control screening solution is a patented, peer reviewed, FDA 510(k) approved, with more patents pending.
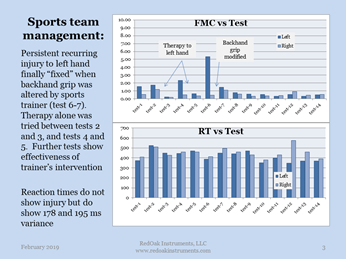
In the example shown, a tennis player developed a problem with her left hand. ROI’s tests presented the problem, and the trainer addressed the situation. Note that the traditional physical measurement of the reaction times does not indicate a problem at all.
Business Model
Revenue Model
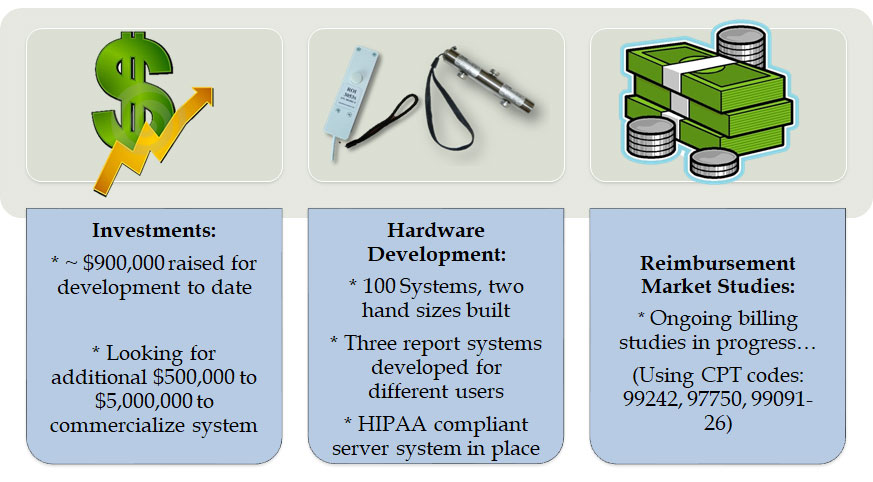
Initial sales or leasing of the equipment will account for initial revenue. This is approximately $5,000 per device/year.
A monthly service fee is included in this amount to provide remote HIPAA compliant access, maintenance, and software upgrades.
As the company grows, we plan to provide remote testing clinics which will provide tests for walk in patients. Our beta testing has been seeing walk in subjects willing to pay $79 to $129 per test. This has been advertised to encourage parents to have their kids tested when they suspect injury. This has shown to be effective with soccer leagues and community sports leagues. Kiosk locations in Malls and/or pharmacy or grocery stores could also be used.
A second-generation sensor system with additional capabilities is already under development and is intended to enhance the capabilities of medical professionals, allowing them to gather more in-depth data. This will give the medical practitioners the ability to better diagnose injuries ranging from traumatic brain injury to Alzheimer’s, and will be available for ~$20,000/yr.
Customer Values
Sports trainers, coaches, physical therapists, sports clinics, wellness clinics, and doctor’s offices can conduct and evaluate the tests, and then charge a fee through a capitated service. Three CPT codes have been successfully used for reimbursements in our beta trials with a minimum reimbursement of $63 per test.
Market
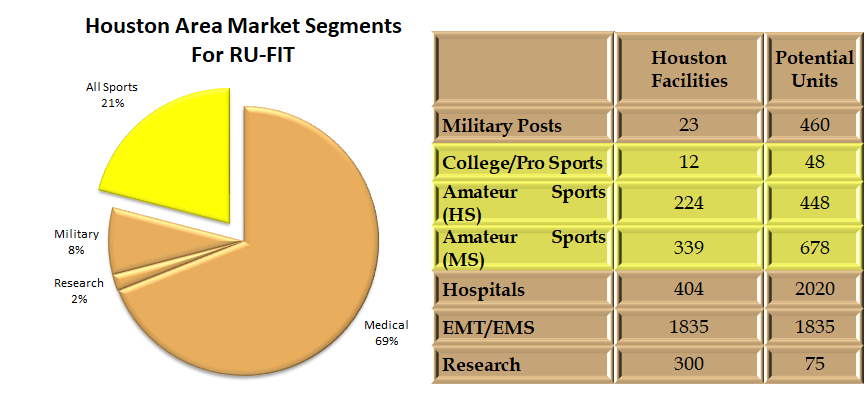
Our initial customers are sports teams, schools, and small medical clinics (PTs, chiropractors and acupuncturists); we intend to expand to larger clinics and hospitals within 12 months. We plan to have sales of the units, and the monthly service fees for server access and updates, making Red Oak Instruments cash positive with 3% penetration of the Houston market. We presently have beta units in Montana and Virginia as well as the units in our hometown of Houston.
Competitive Landscape
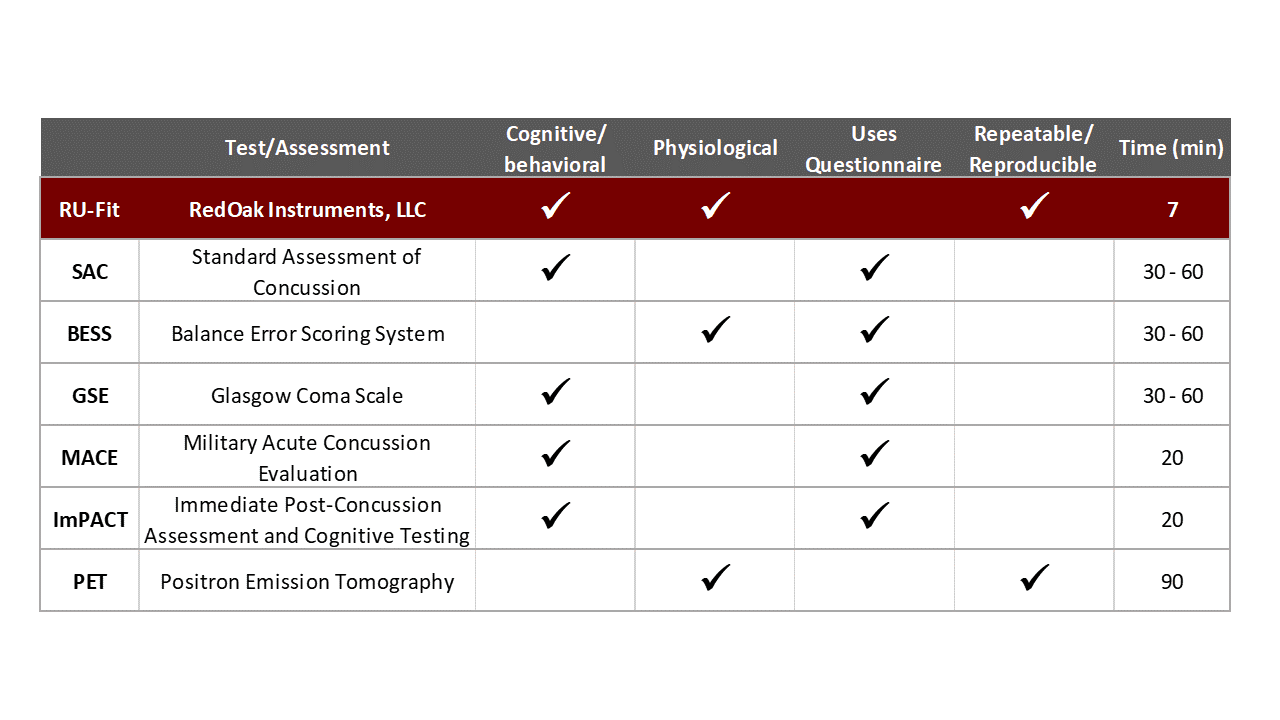
Most of the competitive testing presently available is subjective and requires a trained medical practitioner to accurately administer the test. This is not how it is done in practice since medical staff are expensive. Red Oak Instrument’s tests are fast, objective, and may be administered by anyone after a one-hour training program.
Our tests are repeatable - if the same person runs a second test, you get the same results.
Our tests are reproducible, different people testing the same person get the same results. Our only competitor who can claim this would be a PET scan (positron emission tomography) and they are expensive.
Progress
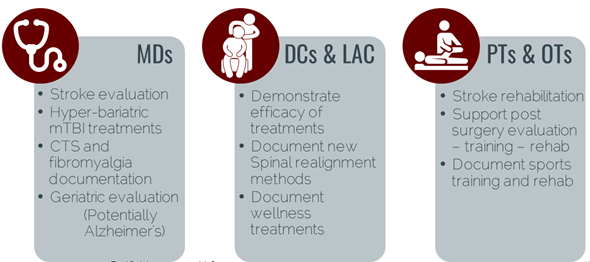
Beginning in 2008, Red Oak Instruments’ beta units have been tested and used by orthopedic surgeons (CTS), psychologists (PTSD/mTBI), and epidemiologists (mTBI, fibromyalgia, Alzheimer’s). Our mTBI technology has been published in peer reviewed papers and presentations have been made at State and National brain injury conferences.
To further market acceptance, early adopters are encouraged to publish their results in papers on mTBI in appropriate sports/training journals. Acceptance in schools (sports applications) are being pursued through the Parent/Teacher organizations, similar to the defibrillator campaign. Parents want assurance their children are safe and well.
In addition, 100 testing systems have been constructed, calibrated and are ready for commercialization.
Publications & Proceedings
- Lefeber, D.J., Li, J., Smith, E.A., Paske, W.C.: (2018) "Can Acupuncture Therapy be Quantified?" The Journal of Acupuncture and Oriental Medicine, 5(4) 16-22.
- Karasch, R.A., Brown, F.A., Smith, E.A., and Paske, W.C.: (2015) “Objective Screening Parameters for Training-Rehabilitation of Sports Teams”, J Clinical Engineering, 40(4) 210-219.
- Mireles, M.C., Jensen, V.N., Myran, L.B., and Paske, W.C. (2013). "Physiological-Cognitive Biomarker for the Presence of Concussion/mTBI". Journal of Clinical Engineering, 38 (4), 191-195.
- Mireles, M.C. (December 2011). "Detection and Prevention of TBI and PTSD: Key Strategy for Continuum of Care and Total Force Fitness." Military Healthcare Convention & Conference Advancing the Continuum of Care Keynote-TBI Spotlight. San Diego, California.
- Mireles, M.C. (June 2011). "Noninvasive Physiological Screening for Mild Traumatic Brain Injury: Case Reports." Military Healthcare Convention & Conference Advancing the Continuum of Care. San Antonio, Texas.
- Mireles, M.C., & Paske, W.C. (April 2011). "Detection and Monitoring of Subconcussive Sequela Based on Non-Invasive Physiological Measurements: Case Reports." Brain Injury Association of Texas 27th Annual Conference. Austin.
- Mireles, M. C., & Paske, W. C. (June 2010). "Is there a physiological difference between mild Traumatic Brain Injuries (mTBI) and Post-Traumatic Stress Disorder (PTSD) among US Veterans?" Brain Injury Association of Texas 26th Annual Conference. Austin.
- Mireles, M. C., Miller, J. A., & Paske, W. C. (2010). "Noninvasive Physiological Screening for mTBI: Preliminary Findings of a Case Study." Journal of Clinical Engineering, 35 (1), 39-45.
- Mireles, M. C., & Paske, W. C. (April, 2009). "mTBI Screening Based on Functional Assessment of Fine Motor Control." Brain Injury Association of Texas 25th Annual Conference. Austin.
- Mireles, M. C., Miller, J. A., & Paske, W. C. (2009). "Misdiagnosis of Carpal Tunnel Syndrome: A Misclassification or Error of Omission." Journal of Clinical Engineering, 34 (2), 99-102.
- Mireles, M. C., Paske, W. C., & Smith, E. A. (May 2008). "Misdiagnosis of Carpal Tunnel Syndrome (CTS): A Systematic Misclassification or Error of Omission." American Medical Informatics Association, Diagnostic Errors in Medicine Conference, (p. 38). Phoenix.
- Paske, W. C. (2008). "mTBI Overview: State of the Art for mTBI Detection/Diagnosis." Houston: Red Oak Instruments. LLC.
- Metzger, C. L. (2006). "Sensokinetogram functionality measurement as a diagnostic aide in carpal tunnel syndrome." ASSH Correspondence News, 68.
- Paske, W. C., Metzger, C. L., & Sutherland, J. M. (2005). "Biomechanical hand-functionality measurement system." Review of Scientific Instruments, 76, 054301(1-9).
Team
Red Oak Instruments is run by a lean team of researchers, developers, optimizers and marketers. The combination of our unique and diverse backgrounds allows us to develop and continually adapt cutting edge technology to meet the needs of the sports medicine community.
Mr. Behlmann was brought on board to develop business strategy and sales, secure intellectual property, oversee regulatory compliance, licensing, FDA, multi-state requirements and sales infrastructure to promote and market the technology. He holds his JD from St. Mary’s University School of Law, San Antonio, Texas, he is a Member, Securities Law Committee, Texas Bar Association; and Missouri Bar Association, Former Member Fixed Wireless Access Consultative Committee, and UK Radio Communications Agency. He holds a BS/BA Business Administration, Concentrations in Finance & Accounting – St. Louis University, St. Louis, Missouri, and CPA – Missouri
Dr. Paske is a Co- Founder of ROI and inventor of the fine motor control measurement technology used by the company. He holds an MS degree in nuclear physics and his PhD in Atomic and molecular physics. He has twenty-five years’ experience with university and large industry research and development and is an expert in systems analysis. Dr Paske has been an innovative and inventive problem solver with 10 US and 24 foreign patents and 60 publications / analysis papers / manuals have been contributed to the scientific community over the past twenty years. His patents range over topics as diverse as electrical transmission problems to nuclear logging tools, to mechanical / medical instrumentation. Many of his patents have been carried from inception to successful commercialization. He has provided proprietary contractual consulting work for Medical professionals on research problems dealing with statistical analysis, physical measurement problems and general protocol methodology.
David Paske attended the University of Tulsa where he earned his Bachelors in Mechanical Engineering. Here he excelled in Engineering Design and Material Science and developed an interest in bioengineering. During his senior design project, he received the opportunity to work alongside other engineers to develop a flaw detection system utilizing hall sensors to detect magnetic flux leakage. This allowed David to augment his teamwork, time management and design skills, but above all allowed David to demonstrate his ability to think outside the box to develop a solution to a difficult problem which he carried throughout his career. Since then, David has worked to redesign the hand sensor to make it more efficient, cheaper to build and more robust. He has gone from designing the equipment to now running the operation on a daily basis.
LTC Paske is a 23-year US Army Veteran and results based leader with the vision and the tenacity to bring this technology to the forefront. Upon realizing the potential value of this technology Chuck has become the driving force behind bringing this technology's potential value to our society through youth Athlete Concussion screening, and through our soldiers/veterans as a diagnostic/detection aid for separating mTBI and PTSD before treatment. with over 30 years’ experience within the Human Resources industry and, over 10 years in Time-Resolved Force Technology™ product design and development, Chuck is the foremost authority on the business functionality screening market and today’s legal and social interface with occupational safety and health, risk management, and the Workers’ Compensation industry. Chuck delivers leadership, motivation, enthusiasm, dedication and passion to this project.
David has studied Electrical Engineering at the University of Houston and holds degrees in Computer Science, from the University of Houston – Downtown, and Automotive Technology, from Wharton County Junior College. While attending school for Computer Science, he conducted research in artificial intelligence and image processing and has written several papers in those areas. David has experience in C++, C#, Objective C, Java, JavaScript, PHP, Pascal, SQL, and LabVIEW. In terms of types of applications, he has experience building graphical (3D) applications, web apps, user interfaces, data storage, data processing and analysis programs.
Daniel has an academic background in Mechanical Engineering and Computer Science, and holds a Bachelor of Science degree in Computer Science from the University of Houston - Downtown. His career in the IT industry started in 2000 while in college at the University of Oklahoma. Daniel’s industry experience ranges from managing a large Professional Services team serving over 45,000 user accounts, to helping create and optimize service offerings for small business-to-business IT firms. Since graduating from UHD, he has devoted his time to software development and is an experienced full stack developer with expertise in the .NET languages, JavaScript, AngularJS, and NodeJS.
Advisors
Dr. Swatzyna is a clinical researcher who has published and presented over 70 studies including 15 publications and two book chapters. He is a popular conference speaker throughout the United States and around the world, with international presentations in over eight countries England, Denmark, Italy, Canada, The Netherlands, Germany, and Australia.
Elizabeth A. Smith, PhD founded the non-profit CRG Medical Foundation for Patient Safety (dba Community Medical Foundation for Patient Safety) in 2003. Her expertise is in health care, human factors, and organizational science. Dr. Smith has 50 years of fact-based academic knowledge and selected content of peer-reviewed publications on productivity, motivation, creativity, and business. Faculty positions: 1) Air Force Institute of Technology, F.E. Warren Air Force Base (1969-1970), 2) Houston Baptist University (1977-1979), and 3) Rice University (1989). Organized, coordinated, and evaluated cancer education grants awarded to M.D. Anderson and published two books on rehabilitation (McGraw-Hill Book Company, New York) (1970-1976). Instructor for Oil & Gas Consultants, International, and presented three-day upper management seminars designed to monitor productivity and increase motivation (1980-1984). Designed and coordinated a “teaching-how-to-teach” curriculum, UT Medical School, Houston (1979-1980). Guided and supported three healthcare non-profits and was an active board member of two science-based research organizations (1976-2020). Was Vice President of a Texas-based independent oil company, monitored production and cash flow (1979-2017). Long-standing Board member of Red Oak Instruments, LLC and currently Advisory Board member.
Karen A Smith graduated from the University of Texas in Austin. She served as a board member of an independently owned corporation for over 30 years, a committee member to various Houston organizations for 27 years and a volunteer to several non-profits groups for 15 years. She developed results driven solutions integrating the benefits of past, present and future. Managed the corporation through the market cycles while maintaining cash flow, analyzing budgeting factors and implementing cost saving for future success. Advised and guided the decision making process for continual business development by asking fact based questions for maximum benefit and enhanced project results by minimizing time, money and effort. Coordinated and managed daily processes and procedures for repeat customer satisfaction and retention. A passionate leader and creative team player that believes all is possible.
Kathleen Paske a comprehensive marketing and operations specialist. She co-founded Vicina Ventures, a boutique real estate investment and lending firm, in 2019. Her experience focuses on an analytics backed and value add approach to strategic planning and go-to-market implementation for fortune 500 and start up companies alike. In addition to ROI, Kathleen currently sits on the Board of Advisors for Hamilton at your Service. She holds a BBA - marketing and MS - business analytics from the McCombs School of Business.
Dr. David E Martinez is a qualified Doctor of Chiropractic. He specializes in chiropractic in Hampton, VA and has over 11 years of experience in the field of medicine. He graduated from his medical school with his medical degree in 2009. Dr. David Martinez is board certified in both Virginia and Texas. He also holds a BA – Health and Wellness and a BS – Anatomy, and is a published author on the topics of concussions.
Mr. Lefeber leads the research and practice of integrated health care and wellness to introduce and educate patients and families the concept of holistic medicine and the appropriate use of complementary and alternative medicine. A graduate from the American College of Acupuncture and Oriental Medicine (ACAOM), with a Masters in Acupuncture and Oriental Medicine, he has been inducted into the international research society, Sigma Xi, has 8 academic publications in different medical journals, and has given research presentations in the Texas Medical Center and International Conferences for Acupuncture and Oriental Medicine.
Use of Proceeds
If the offering's maximum amount of $1,000,000 is raised:
| Use | Value | % of Proceeds |
|---|---|---|
| Compensation for managers | $100,000 | 10.0% |
| Legal and compliance | $25,000 | 2.5% |
| Software Development | $94,250 | 9.4% |
| Marketing and sales | $95,000 | 9.5% |
| Development of tech support | $94,000 | 9.4% |
| Manufacturing | $122,500 | 12.3% |
| FDA Legal/patent support | $65,000 | 6.5% |
| Office Expenses | $125,250 | 12.5% |
| Rent/Utilities | $30,000 | 3.0% |
| Staff support/insurance | $50,000 | 5.0% |
| New Technology | $25,000 | 2.5% |
| Office Staff | $125,000 | 12.5% |
| Intermediary fees | $49,000 | 4.9% |
Terms
This number includes all funds raised by the Company in this round on Netcapital. This is an offering of Membership interest units, under registration exemption 4(a)(6), in RedOak Instruments, LLC. This offering must reach its target of at least $10,000 by its offering deadline of April 23, 2021 at 12:59am ET. If this offering does not reach its target by the offering deadline, then your money will be refunded.
If the offering is successful at raising the maximum amount, then the company’s implied valuation after the offering (sometimes called its post-money valuation) will be:
Pitch Deck
Financials
These financial statements have been reviewed by an independent Certified Public Accountant.
SEC Filings
The Offering Statement is a formal description of the company and this transaction. It’s filed with the SEC to comply with the requirements of exemption 4(a)(6) of the Securities Act of 1933.
We’re also required to share links to each of the SEC filings related to this offering with investors.
Understand the Risks
Be sure to understand the risks of this type of investment. No regulatory body (not the SEC, not any state regulator) has passed upon the merits of or given its approval to the securities, the terms of the offering, or the accuracy or completeness of any offering materials or information posted herein. That’s typical for Regulation CF offerings like this one.
Neither Netcapital nor any of its directors, officers, employees, representatives, affiliates, or agents shall have any liability whatsoever arising from any error or incompleteness of fact or opinion in, or lack of care in the preparation or publication of, the materials and communication herein or the terms or valuation of any securities offering.
The information contained herein includes forward-looking statements. These statements relate to future events or to future financial performance, and involve known and unknown risks, uncertainties, and other factors, that may cause actual results to be materially different from any future results, levels of activity, performance, or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties, and other factors, which are, in some cases, beyond the company’s control and which could, and likely will, materially affect actual results, levels of activity, performance, or achievements. Any forward-looking statement reflects the current views with respect to future events and is subject to these and other risks, uncertainties, and assumptions relating to operations, results of operations, growth strategy, and liquidity. No obligation exists to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
More Info
Updates
- May 12, 2025It is management’s intent to terminate...
- Aug 4, 2023Houston-based biotech company Red Oak...
- Apr 23, 2021Primary offering finalized, selling units
- Mar 12, 2021Recently published research in Alzheimer’s &...
- Mar 3, 2021ROI has completed a webinar discussion on...
- Feb 19, 2021ROI’s unique technology has enabled a Houston...
Ask a Question
Proofread your comment before submitting: once it's posted, you can’t edit or delete it. Investors are advised to review our Discussion Board Policy before submitting a comment. For the fastest help with the web site, email help@netcapital.com instead of commenting.
