None currently available
None currently available
30,269
$0.80 — $5
Introduction
Phoenix PharmaLabs (PPL) is a preclinical drug discovery company dedicated to the development of potent, non-addictive treatment for pain and treatments for addiction. PPL’s lead drug has demonstrated in animal studies that it is a very potent painkiller (10x more potent than morphine), and is non-addictive, does not produce withdrawal, does not cause death from overdose (even at 350x the analgesic dose) and causes no constipation (even at 100x dose). It has also demonstrated effectiveness in treating opioid addiction and cocaine addiction. Approximately $3 million of grant funding has been awarded to the company by the US Army and the NIH. Those funds will enable the company to advance this drug into human clinical trials.

Problem
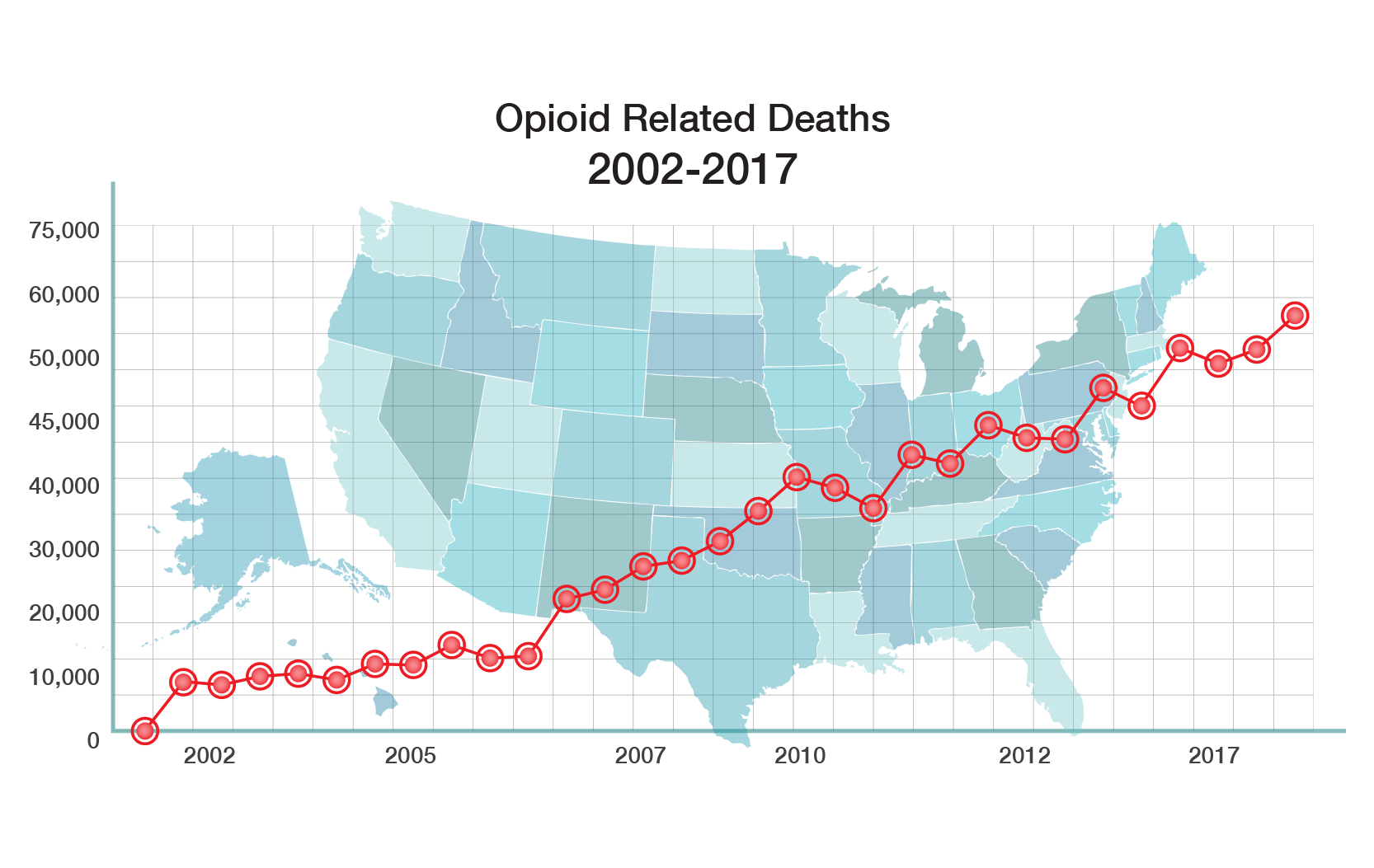
There is a significant need for non-addictive potent pain relief. Opioids are the most widely prescribed drugs for moderate to severe pain. All current opioid drugs bind to the mu receptor in the brain and then aggressively stimulate that receptor. Activation of that receptor produces a euphoric “high”, which is what leads to abuse and addiction. Activation of the mu receptor also causes other serious side effects such as withdrawal, constipation and death from overdose.
The CDC has declared that “addiction to prescription opioids is a major public health problem that is getting worse and getting worse rapidly.” The opioid epidemic is killing almost 45,000 Americans a year and costs the U.S. economy $78.5 billion a year, according to a new government study.
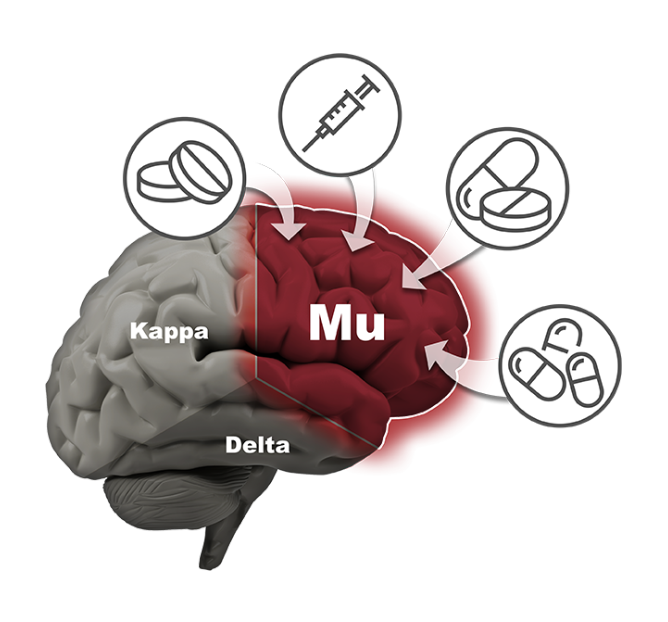
Solution
Phoenix is developing a very potent pain reliever that has been demonstrated in preclinical animal studies to show no signs of euphoria (which is what leads to abuse and addiction), no death from overdose (even at 350x dose), no physical dependence or withdrawal, and no constipation (even at 100x dose). The company believes its family of drugs will provide a safe alternative to the dangerous and addicting opioid drugs currently available on the market. They may also provide an excellent treatment for various drug addictions.
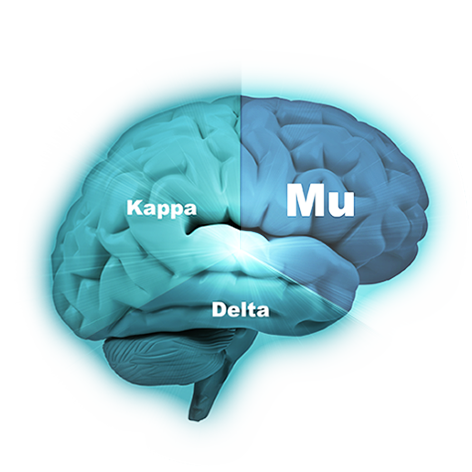
The company’s lead compound, PPL-103, binds strongly to all three opioid receptors (mu, kappa and delta) and then just partially stimulates those receptors in a much more balanced manner. PPL-103 stimulates the mu receptor only slightly (about 10%) with higher, but not full, stimulation of kappa and delta. That partial stimulation derives analgesic benefit from all three receptors, but it is not sufficiently strong to produce the serious opioid side effects associated with any of the receptors. This is unique and to our knowledge is the only compound in development with this profile and ability.
PPL-103 has clearly demonstrated in various animal studies that it does not produce euphoria (or dysphoria) and will not lead to addiction. Research has shown that there is an extremely high correlation between these animal studies and Human Abuse Liability studies and other indicators that measure the potential for abuse and addiction in humans.1

Since PPL-103 does not precipitate withdrawal in Non-Human Primates (NHP) we believe it offers very promising use for addiction therapy as a preferred substitute for methadone, buprenorphine and Suboxone, since those drugs are, in and of themselves, addicting opiates that addicts typically have to remain on for life. Furthermore, recent animal studies conducted at the Torrey Pines Institute for Molecular Studies under the supervision of our Chief Neuropharmacologist, Dr. Larry Toll, have shown that PPL-103 has the potential to prevent relapse in people recovering from cocaine addiction.
PPL-103 has been substantially studied in animals relative to problems that are likely to occur with opioids. A vast amount of opioid testing data is available concerning the transition of effects of pure opioid compounds from animals to humans. Thus there is a high level of confidence that this compound will be safe, effective and beneficial for humans.
1O’Connor EC, Chapman K, Butler P, Mead NM. (2011) The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 35:912-938
Market
The market for opioids is estimated to reach $34.96 billion by 2025 and is growing at a rate of about 5% per year.* If opioids were not plagued by such serious side effects this market segment would likely be much larger.
* Opioids Market Size, Share & Trends Analysis Report 2018 - 2025 by Research and Markets
Competitive Landscape
All of the opioids in use today are mu opioids. They produce a euphoric “high”, and consequently they are addicting and dangerous. The only exception to this are some peripherally-acting mu opioids that are under development that could potentially be effective in treating pain locally in certain parts of the body. Mu / NOP (Nociceptin) receptor compounds in development could have potential.
Some other pain drugs, such as non-steroidal anti-inflammatories, are on the market and others, such as cannabinoids, are under development, but none of these are as potent as opioids for relieving moderate to severe pain.
Business Model
The strategic objective of the company is to enter into license agreements with appropriate market leader(s) that have the resources to maximize the market potential of PPL’s drugs. Such licenses would likely be for treatment of pain, opioid addiction, cocaine addiction and animal health.
The company has held discussions with several leading pharma companies that have a strategic focus on pain. They all expressed serious interest in licensing PPL-103 for pain after it has entered human clinical trials. PPL is currently advancing PPL-103 through preclinical studies and then to proof of concept (Phase IIa) in humans. At or before that point is reached (probably in about three years or so) the company expects to license PPL-103 to one of those companies for pain. Additional licenses for addiction therapy and animal health are also areas for potential license agreements.
The following are examples of disclosed terms* for previous license deals for various drugs for pain including Licensor/Licensee; Stage; Upfront Fees; Milestone Payments; and Royalties as a % of Net Revenues (sometimes not disclosed):
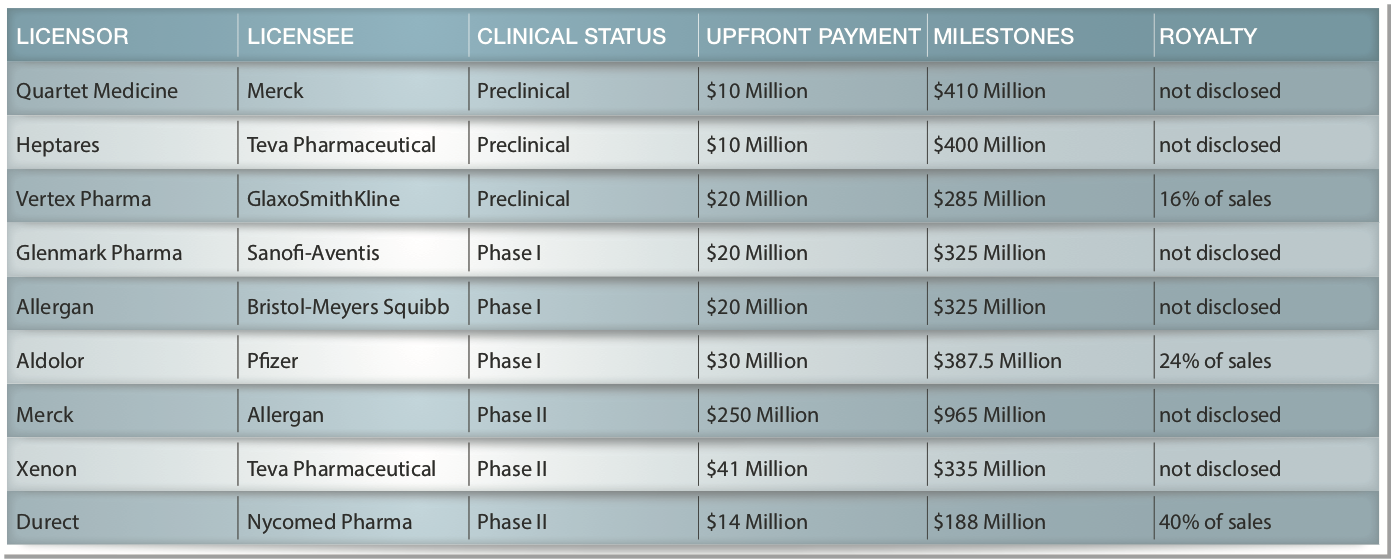
There is no assurance that PPL will enter into a similar license agreement. However, if PPL-103 performs in humans similar to the way it has in animals we believe its license potential would be very significant.
* Source: Selected license agreements with disclosed data since 2005 from Cortellis Deals Intelligence / Thomson Reuters Life Sciences (formerly Deloitte Recap) database
Progress
Unlike a pharmaceutical company, our “customer” is not the consumer. Rather, we seek to license our drug to a large company who will market it to the consumer.
We have developed a strategy that focuses on licensing our lead drug, PPL-103, or a variant thereof, to one or more large pharmaceutical companies for use in pain relief and opioid and/or cocaine addiction therapy.
Licensing relies on strong patent protection. A Composition of matter patent (Patent No. 8,987,293) for PPL-103 was issued in the US in March, 2015 and PPL-103 has since received patent approval in major countries around the world. This patent also covers Methods of synthesis as well as Use for treatment of opioid and cocaine addiction therapy as well as pain, and Use for treatment of pain in animals.
In addition to our obvious potential partners in the pharmaceutical/pain/addiction space, we also plan to target the animal health market. PPL-103 is likely to be either unscheduled or scheduled at a low enough level for pet owners to be permitted to administer it to their pets at home. Many pets are currently in moderate to severe pain and their owners cannot get access to safe, potent pain medications.
Our strategy for out-licensing is to advance PPL-103 as quickly as possible into human clinical trials, through Phase I and into Phase II to Proof of Concept (POC) in humans at which point it will be ideally positioned for out-licensing. It is possible, however, that we could enter one or more license agreements or an acquisition or an IPO before that point is reached. At any one of those points investors could realize a return on their investment, although there is no assurance that any of those exit points will be reached.
Success


The company was awarded a $2.7 million grant from the US Army Medical Research and Material Command (USAMRMC) which will fund the advancement of PPL-103 into human clinical trials. The company has strong support from scientists at the USAMRMC.
The company also received a $185,000 grant from the NIH / NIDA (National Institute for Drug Abuse) to study PPL-103 for potential use as a cocaine addiction medication. It has already been demonstrated that the drug is effective at blocking relapse from cocaine addiction in rodents. Currently there are no medications that are effective for treating cocaine addiction.
Press mentions:
- US Department of Defense grants funding for development of non-addictive opioid
- How to fight drug addiction: the shift to prescription drugs
- Phoenix PharmaLabs Exec Describes Novel Non-Addictive Opioid
- Research Declares Phoenix PharmaLabs' Opioid PPL-103 Safe
- Additionally, the news media is beginning to learn more about PPL-103. Here is a link to a story last November in STAT
- One Stepfather’s Quest: The Search For An Opioid That Won’t Lead To Addiction
- One Stepfather’s Quest: The Search For An Opioid That Won’t Lead To Addiction
- In Vitro and In Vivo Profile of PPL-101 and PPL-103: Mixed Opioid Partial Agonist Analgesics with Low Abuse Potential
- BioSpace: Phoenix PharmaLabs Announces Investment Offering to Support Additional Research of Non-Addictive Opioid
- Global Banking And Finance Review: Phoenix PharmaLabs Announces Investment Offering to Support Additional Research of Non-Addictive Opioid
KPIs
- Budget
- Schedules for manufacturing scale-up, preclinical and later clinical studies, and FDA regulatory requirements
- Specific manufacturing objectives and related metrics such as cost and lead time for sourcing raw materials, process time, yields, and end-product analytics such as purity and stability. Recently improvements in these metrics have resulted in identified savings of more than $600,000 in projected manufacturing costs next year.
- Successful results of animal studies and human clinical trials. Such studies that have been conducted so far have demonstrated the very promising characteristics of PPL-103 described above as well as a number of related attributes.
- The detailed results of these studies can be seen in the PPL-103Technical Narrative.
Team
- Motivation & Determination - Members of the management team have witnessed firsthand the devastating effects of opioid addiction. The CEO’s stepson has been seriously addicted to opioids for more than 15 years, and other members of the team have friends and family who were addicted and even died from opioid overdose. The team strongly believes that PPL-103 can play an important role in addressing the opioid crisis and is determined to get that drug to market as quickly as possible.
- Scientific Knowledge and Creativity - The company’s scientists are highly regarded in their field. They developed extensive expertise in medicinal chemistry and neuropharmacology at institutions such as Stanford Research Institute (SRI) and Torrey Pines Institute for Molecular Studies. Prominent scientists at leading institutions such as the National Institute on Drug Abuse (NIDA), Virginia Commonwealth University (VCU), the University of Michigan, and the Rockefeller University have also contributed to the company’s drug development technology.
- Lean Business Practices - The team is experienced in lean business practices. The company has a track record of accomplishing a great deal on a lean budget so far and intends to continue that discipline in the future.
Bill Crossman is a senior management professional with domestic and international experience as CEO, CFO and other senior management positions in enterprises ranging from entrepreneurial start-ups to Fortune 100 level companies. Mr. Crossman has a proven track record of successfully commercializing emerging technologies. He has assisted numerous early-stage companies to refine business strategies, commercialize new products, raise capital, license technologies, scale revenues and production, and expand into global markets. Crossman also has expertise in implementing lean business practices and continuous improvement strategies. Bill holds a BS degree from the U.S. Merchant Marine Academy at Kings Point and a MBA from the Haas School of Business at the University of California – Berkeley.
Dr. Lawson is an expert in medicinal and synthetic organic chemistry. As a senior Medicinal Chemist and Project Manager at Stanford Research Institute (SRI International) for 20 years, he headed the Neurochemistry R & D Group with responsibilities for the discovery and development of new compounds in neuroscience including analgesics, anti-convulsants, anxiolytics, and stroke therapeutics. While at SRI, Lawson collaborated for ten years with Dr. Toll, Chief Neuropharmacologist and Board Member of Phoenix PharmaLabs (see below), on analgesic drugs under NIH grant funding. During this period, Dr. Lawson discovered the initial class of opioids capable of relieving pain without the typical side-effect problems of morphine-like opioids. After leaving SRI, Lawson founded Phoenix PharmaLabs, Inc. for the purpose of renewing the development of non-addicting opioid compounds. John holds a BS degree with Phi Kappa Phi honors from Iowa State University and a Ph.D. from the University of Oregon. He was also a Postdoc. fellow at Syntex Corp.
Mr. Chou is a serial entrepreneur and founding partner of Spectra Consulting Group where for over 25 years he has performed consulting on growth issues in emerging business development, including specialized consulting in strategic planning, capital acquisition, capital structures, organization re-engineering, process development, and dispute mediation. He specializes in start-up consulting for technology, life science, and consumer product companies. Mr. Chou’s corporate experience includes serving as a Chief Financial Officer, Controller, and as a CEO. He currently serves as an officer of several operating companies and sits on various Boards as a director. He has participated in architecting numerous capital structures and has developed strategies for development-stage enterprises that have produced significant debt and/or equity investment. Mr. Chou’s clients are located across the western United States.
Dr. Toll is currently Professor, Department of Biomedical Sciences, Charles E. Schmidt College of Medicine, Florida Atlantic University. He is also President of the International Narcotics Research Conference. Formerly he was Director of Neuropharmacology, Torrey Pines Institute for Molecular Studies, and prior to that he was Director of the Neuropharmacology Department at SRI International where he conducted basic and translational research in the fields of pain and addiction. An author of more than 130 peer-reviewed scientific papers, he is a recognized expert and leading researcher in the field of neuroscience, particularly in relation to addiction neurobiology and the pharmacology of drugs with potential addiction liability, such as opiates. Dr. Toll is a co-discoverer of the “nociceptin” opioid peptide and was part of the Opiate Research team at SRI that first researched potential non-addicting opioids. Dr. Toll has performed numerous selected assessments of neurochemical drugs for the National Institute on Drug Abuse (NIDA). As part of NIDA’s Opiate and Cocaine Treatment Discovery Programs he tested a very large number of compounds for affinities and activities at the three opioid receptors, 5 dopamine receptors, several 5-HT receptors, PCP receptors and sigma receptors. In conjunction with this project, Dr. Toll and his team published the definitive in vitro profile of opioid- and cocaine-related ligands. His lab has collaborated with many medicinal chemists to characterize the activity of a large number of compounds, including PPL’s compounds. Larry holds a BA degree in chemistry from the University of California, San Diego and a Ph.D. in biological chemistry from the University of California, Los Angeles as well as a Postdoc. in biological chemistry at the University of California, Los Angeles and a Postdoc. in pharmacology at Johns Hopkins University.
Mr. Tew brings over 25 years of professional bioscience sales, marketing and business development leadership experience to the company as a sales, marketing and development executive with American Hospital Supply, CooperVision, and Alcon. Throughout his career Mr. Tew championed many successful sales and product initiatives. In addition, Chris co-founded HealthWare Management Company, a healthcare software company, which was sold at a profit to Global Software. Chris holds a BA degree in mass communications from Brigham Young University.
Advisors and Investors
Dr. Kreek is Senior Attending Physician and Patrick E. and Beatrice M. Haggerty Professor, Head of Laboratory of Biology of Addictive Diseases, The Rockefeller University. She is the recipient of numerous professional awards including: Betty Ford Award, Association for Medical Education and Research in Substance Abuse, 1996; Specific Recognition Award, Research in Science of Addiction, Executive Office of the President – Office of National Drug Control Policy 1998; R. Brinkley Smithers Distinguished Scientist Award, American Society of Addiction Medicine, 1999; Nathan B. Eddy Memorial Award for Lifetime Excellence in Drug Abuse Research, College on Problems of Drug Dependence, 1999; Fellow, New York Academy of Sciences, 2000; Columbia University College of Physicians and Surgeons Alumni Association – Gold Medal for Distinguished Achievements in Academic Medicine, 2004; Marian W. Fischman Memorial Lectureship Award, College on Problems of Drug Dependence, 2005; President, International Narcotics Research Conference 2002-2006. Dr Kreek holds a BA in Chemistry from Wellesley College where she was selected as a member of Sigma Xi and Phi Beta Kappa and where her thesis work allowed her to be named a Durant Scholar; she received her MD from Columbia College of Physicians and Surgeons.
Dr. Levy is Principal, BioPharma Consulting Services. Dr. Levy has extensive experience in all aspects of organic and medicinal chemistry and has designed and developed syntheses for novel chemical entities from various chemical and pharmaceutical classes. Most recently Dr. Levy was the Director of Synthetic Chemistry at Intradigm Corporation. He is author and editor of three books on organic chemistry. Dr. Levy received his academic degrees from the University of California - Berkeley (B.S., 1987) and the Massachusetts Institute of Technology (Ph.D., 1992)
Dr. Gad is Principal, Gad Consulting Services, a twenty year old consulting firm with over 300 pharmaceutical companies as clients. He has more than 35 years of broad-based experience in toxicology, drug development, statistics and risk assessment and has authored or edited 44 published books in these fields. He is the recipient of the American College of Toxicology Lifetime Contribution Award. He has direct involvement in the preparation of INDs (96 successful to date) and clinical databases for Phase I and II studies. He has consulted for FDA, EPA and NIH, and has trained reviewers and been an expert witness for the FDA. Shayne holds two BS degrees from Whittier College in Chemistry and Biology, and a Ph.D. in Pharmacology / Toxicology from the University of Texas DABT, ATS.
Dr. Mendelson is Senior Scientist, Addiction and Pharmacology Research Laboratory, California Pacific Medical Center Research Institute and is also Clinical Professor of Medicine at the University of California, San Francisco. He is also a member of the FDA Analgesic Drugs Advisory Committee. He is the former Chair of the California Pacific Medical Center Institutional Review Board. He has been Selected, Best Doctors USA and has frequently served as Section Chair of the American Society of Clinical Pharmacology and Therapeutics. He has specific expertise in the design and supervision of human clinical trials of controlled substances. John holds a BA in Biology from Antioch College and an MD from the University of California, San Francisco.
Dr. Ko is Professor, Department of Physiology & Pharmacology, Wake Forest University School of Medicine
Use of Proceeds
- Additional funding beyond the funds provided by the Army grant will be used to accelerate the scale-up of manufacturing of PPL-103 as well as speed up preclinical and clinical studies without sacrificing quality or reliability.
- Should the company achieve the funding goal of this offering, it intends to use the funds to advance PPL-103 through Phase I human clinical trials. Studies of potential side effects are expected to include Human Abuse Liability (HAL) studies as well as studies of respiration, constipation and physical dependence / withdrawal. The results of these studies are expected to be highly valuable for large pharmaceutical companies to evaluate potential license(s) of PPL-103 and variants thereof.
If the offering's maximum Reg CF allocation of $1,069,999 is raised:
| Use | Value | % of Proceeds |
|---|---|---|
| Working Capital | $1,017,569 | 95.1% |
| Intermediary fees | $52,430 | 4.9% |
If the offering's maximum amount of $3,500,000 across Reg. CF and Reg. D is raised:
| Use | Value | % of Proceeds |
|---|---|---|
| Compensation for Directors, Officers, and Promoters | $758,500 | 21.7% |
| Contract Research Organization - Subcontractors | $1,998,145 | 57.1% |
| Consulting Fees & Expense | $323,355 | 9.2% |
| Legal/IP/Insurance/Misc Overhead | $248,500 | 7.1% |
| Intermediary fees | $171,500 | 4.9% |
Terms
This is a side-by-side offering of Common Stock, under registration exemptions 4(a)(6) and 506(c), in Phoenix PharmaLabs Inc. Up to $1,069,999.47 may be raised under the 4(a)(6) exemption. Netcapital will determine which exemption applies to your investment and notify you before you complete your investment.
The amount raised under the two exemptions must total at least $10,000 by March 30, 2019 at 6:59pm ET. If the total doesn’t reach its target, then your money will be refunded. Phoenix PharmaLabs Inc may issue additional securities to raise up to $3,500,000, the offering’s maximum.
If the side-by-side offering is successful at raising the maximum amount, then the company’s implied valuation after the offering (sometimes called its post-money valuation) will be:
Pitch Deck
Financials
These financial statements have been audited by an independent Certified Public Accountant.
SEC Filings
The Offering Statement is a formal description of the company and this transaction. It’s filed with the SEC to comply with the requirements of exemptions 4(a)(6) and 506(c) of the Securities Act of 1933. Similar information is sometimes offered in a Private Placement Memorandum for 506(c) offerings.
We’re also required to share links to each of the SEC filings related to this side-by-side offering with investors.
Website
Understand the Risks
Be sure to understand the risks of this type of investment. No regulatory body (not the SEC, not any state regulator) has passed upon the merits of or given its approval to the securities, the terms of the offering, or the accuracy or completeness of any offering materials or information posted herein. That’s typical for Regulation CF offerings like this one.
Neither Netcapital nor any of its directors, officers, employees, representatives, affiliates, or agents shall have any liability whatsoever arising from any error or incompleteness of fact or opinion in, or lack of care in the preparation or publication of, the materials and communication herein or the terms or valuation of any securities offering.
The information contained herein includes forward-looking statements. These statements relate to future events or to future financial performance, and involve known and unknown risks, uncertainties, and other factors, that may cause actual results to be materially different from any future results, levels of activity, performance, or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties, and other factors, which are, in some cases, beyond the company’s control and which could, and likely will, materially affect actual results, levels of activity, performance, or achievements. Any forward-looking statement reflects the current views with respect to future events and is subject to these and other risks, uncertainties, and assumptions relating to operations, results of operations, growth strategy, and liquidity. No obligation exists to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
More Info
Updates
- Jul 9, 2024New Phoenix update- you are not on the...
- Jul 12, 2023Phoenix PharmaLabs update: 1. PPL-138 continues...
- Oct 6, 2022Phoenix awarded 8.7m grant from NIDA to study...
- Sep 13, 2021Dr. Lawrence Toll, Chief Neuropharmacologist at...
- Jun 13, 2021NOTICE: The Phoenix PharmaLabs offering will...
- Jun 11, 2021Phoenix PharmaLabs Update: Phoenix recently...
- May 14, 2021Phoenix PharmaLabs Update: At the urging of...
- Jan 29, 2021Thought you would like to see some of the...
- Mar 30, 2019Primary offering finalized, selling shares
Ask a Question
Proofread your comment before submitting: once it's posted, you can’t edit or delete it. Investors are advised to review our Discussion Board Policy before submitting a comment. For the fastest help with the web site, email help@netcapital.com instead of commenting.
