Overview
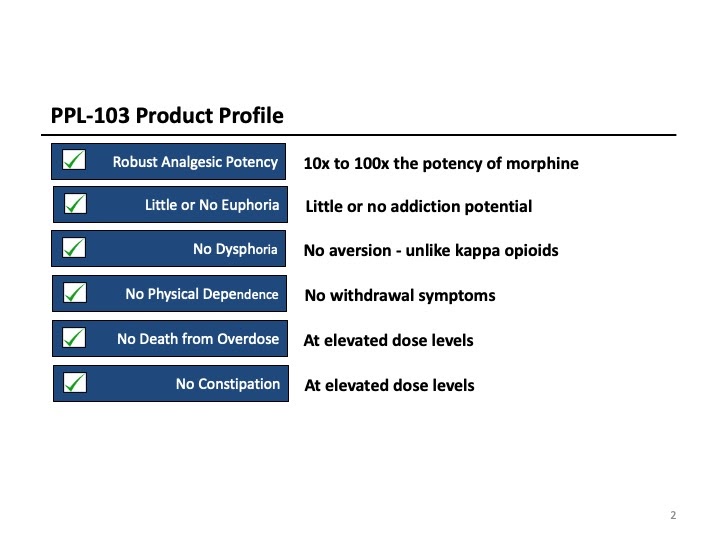
Phoenix PharmaLabs (PPL) is a preclinical drug discovery company dedicated to the development of potent, safe, non-addictive treatments for pain and addiction. PPL’s lead drug, PPL-103, has demonstrated in animal studies that it is a very potent painkiller (10x more potent than morphine), and is non-addictive, does not produce withdrawal, does not cause death from overdose at elevated doses, and causes no constipation. It has also demonstrated effectiveness in treating opioid and cocaine addictions. Approximately $3 million of grant funding has been awarded to the company by the US Army and the NIH, in addition to over $3 million in private capital and investments-in-kind. Those funds are enabling the company to advance this drug into human clinical trials. And recently we acquired the closest competing technology.
New Opportunity
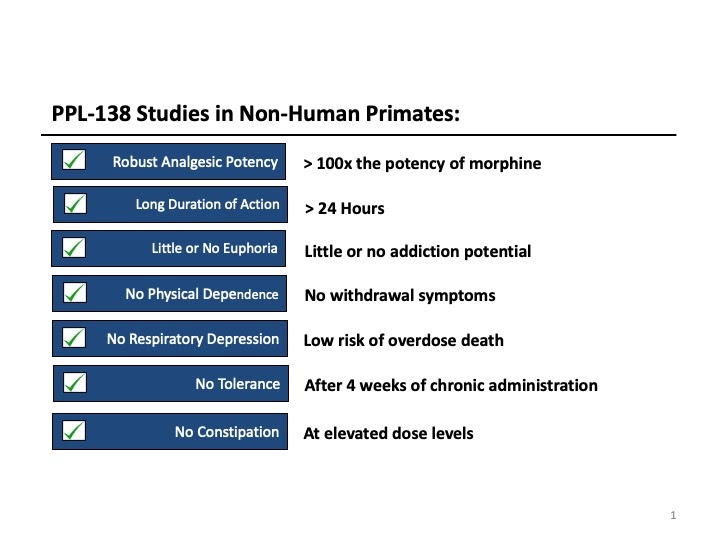
Phoenix recently acquired 25 Nociceptin compounds from the University of Bath (England) including BU10038 (which we now call PPL-138). This compound met all of PPL’s criteria for a Potent, Safe, Non-Addicting Analgesic for Effective Treatment of Moderate to Severe Pain. It has demonstrated long duration of action, effective bioavailability in sublingual administration, and no development of tolerance after four weeks of chronic administration.
Since PPL-138 has such a long duration of action it has excellent potential for development as a non-addicting treatment for chronic pain. Therefore, as these compounds move into human clinical trials we plan to advance PPL-103 for treatment of acute pain and PPL-138 for treatment of chronic pain. Both compounds have also demonstrated promising potential as therapies for opioid as well as cocaine addiction.
Problem
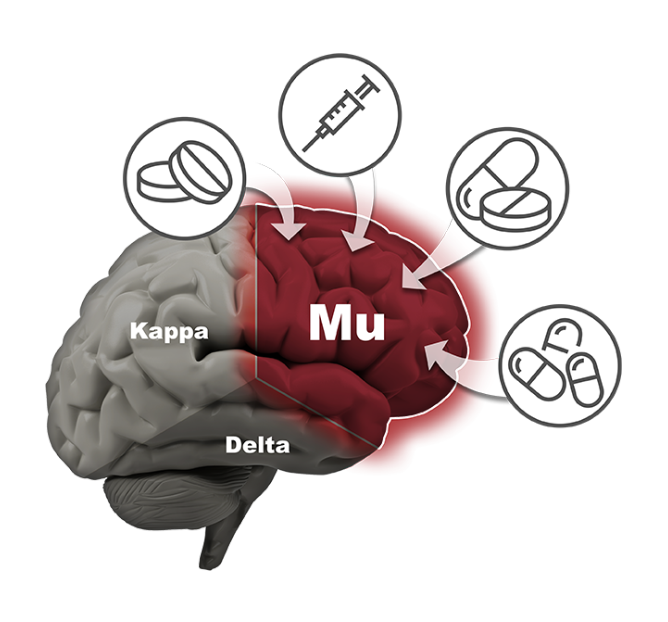
There is a significant need for non-addictive potent pain relief. Opioids are the most widely prescribed drugs for moderate to severe pain. All current opioid drugs bind to the mu receptor in the brain and then aggressively stimulate that receptor. Activation of that receptor produces a euphoric “high”, which is what leads to abuse and addiction. Activation of the mu receptor also causes other serious side effects such as withdrawal, constipation and death from overdose.
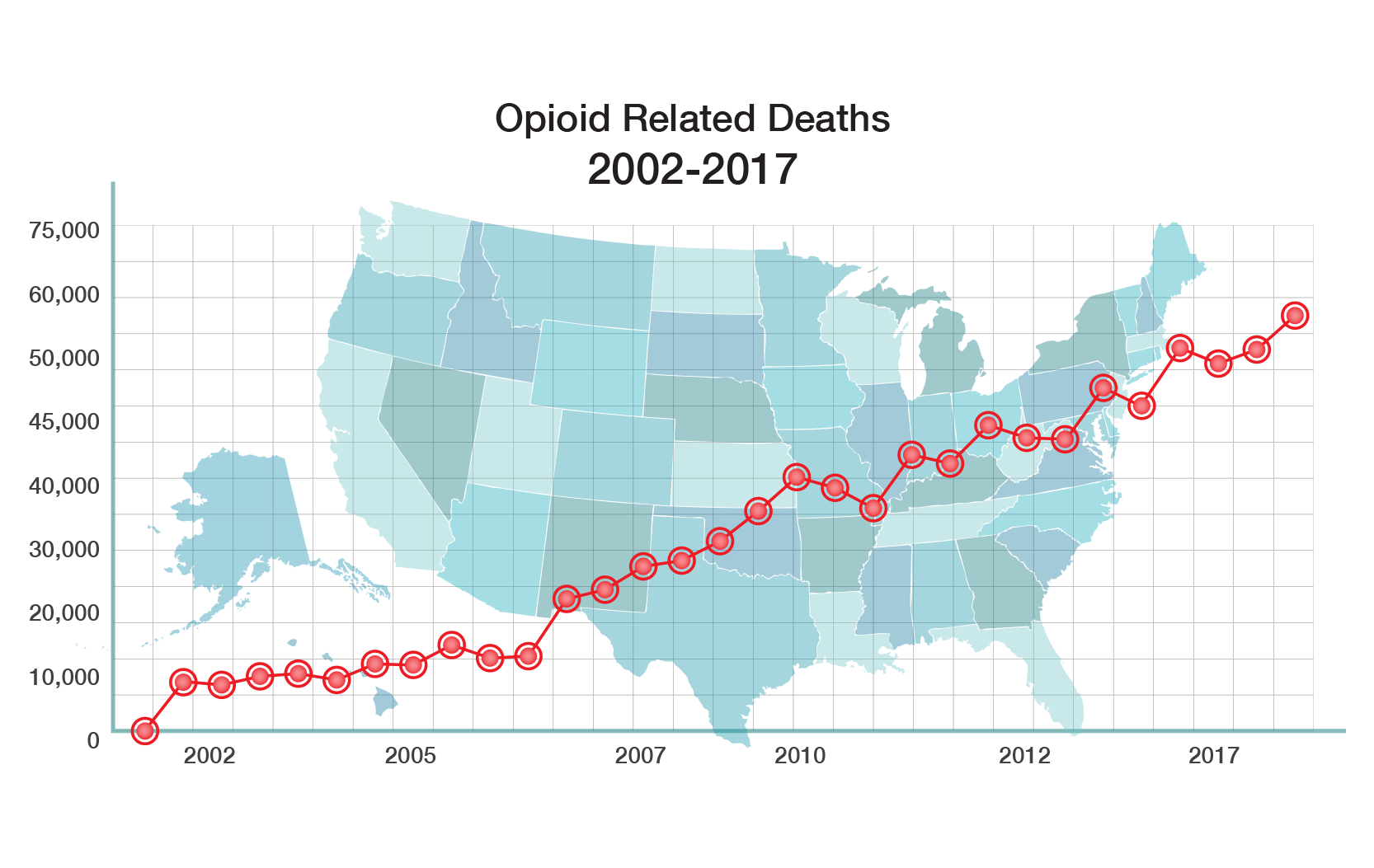
The CDC has declared that “addiction to prescription opioids is a major public health problem that is getting worse and getting worse rapidly.” The opioid epidemic is killing almost 50,000 Americans a year and costs the U.S. economy $696 billion a year, according to the Council of Economic Advisers (CEA).
Solution
Phoenix is developing very potent pain relievers that have demonstrated in preclinical animal studies to show no signs of euphoria (which is what leads to abuse and addiction), no death from overdose, no physical dependence or withdrawal, and no constipation. The company believes its family of drugs will provide a safe alternative to the dangerous and addicting opioid drugs currently available on the market. They may also provide an excellent treatment for various drug addictions.
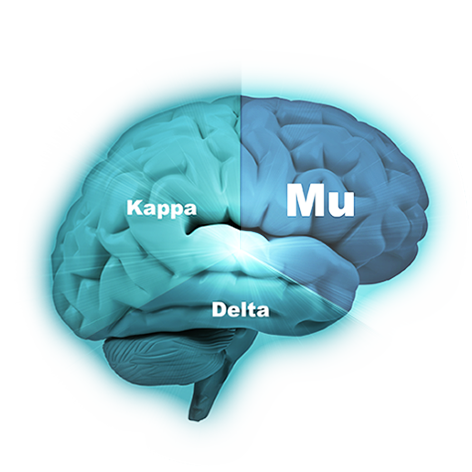
The company’s lead compound, PPL-103, binds strongly to all three opioid receptors (mu, kappa and delta) and then just partially stimulates those receptors in a much more balanced manner. PPL-103 stimulates the mu receptor only slightly (about 20%) with higher, but not full, stimulation of kappa and delta. That partial stimulation derives analgesic benefit from all three receptors, but it is not sufficiently strong to produce the serious opioid side effects associated with any of these receptors. PPL-138 on the other hand uses a different mechanism of action. It partially stimulates the mu and NOP (Nociceptin) receptors. Phoenix’s two very promising compounds using diverse approaches improve the company’s chances of success.
PPL-103 and PPL-138 have clearly demonstrated in various animal studies that they do not produce euphoria (or dysphoria) and will not lead to addiction. Research has shown that there is an extremely high correlation between these animal studies and Human Abuse Liability studies and other indicators that measure the potential for abuse and addiction in humans[1].
Since PPL-103 does not precipitate withdrawal in Non-Human Primates (NHP) we believe it offers very promising use for addiction therapy as a preferred substitute for methadone, buprenorphine and Suboxone, since those drugs are, in and of themselves, addicting opiates that addicts typically have to remain on for life. Furthermore, recent animal studies conducted at the Torrey Pines Institute for Molecular Studies have shown that PPL-103 has promising potential to prevent relapse in people recovering from cocaine addiction.
PPL-103 has been substantially studied in animals relative to problems that are likely to occur with opioids. A vast amount of opioid testing data is available concerning the transition of effects of pure opioid compounds from animals to humans. Thus there is a high level of confidence that this compound will be safe, effective and beneficial for humans.
Fontiers PPL PaperTechnical NarrativeCompetitive Landscape
The company recently conducted a thorough review of its competition for a Potent, Safe, Non-Addicting Analgesic for Effective Treatment of Moderate to Severe Pain. Various drugs were investigated including opioid approaches, cannabinoid receptor drugs, capsaicin receptor drugs, etc. Many analgesics on the market and in development are effective for treating mild to moderate pain, but no drugs are as effective as opioids for treating moderate to severe pain.
A full mu/NOP (Nociceptin) agonist compound is being developed by Grunenthal, a leading German pharmaceutical company that is reported to demonstrate reduced opioid side effects, but the results of self-administration studies have not been released so the degree to which it produces euphoria is unclear. Since it is a full agonist at mu, however, it is likely to produce more euphoria than PPL-103.
Besides PPL-103 the only analgesics that appear to provide potent, safe, non-addicting relief of moderate to severe pain are two partial mu/NOP compounds – and Phoenix just acquired one of them - now called PPL-138 as discussed above. (The other, called AT-121, is being advanced through preclinical studies by Astraea Therapeutics.)
Market
The market for opioids is estimated to reach $34.96 billion by 2025 and is growing at a rate of about 5% per year[2]. If opioids were not plagued by such serious side effects this market segment would likely be much larger since many people in the world are in severe pain without access to opioids because of the addiction liability.
Business Model
The strategic objective of the company is to enter into license agreements with appropriate market leader(s) that have the resources to maximize the market potential of PPL’s drugs. Our first objective is to license these compounds for non-addictive treatment of acute and chronic pain. There may also be opportunities to license PPL compounds for opioid and cocaine addiction therapy, anti-itch and animal health indications.
The company has held discussions and conducted due diligence with several leading pharma companies that have a strategic focus on pain. Most of them expressed serious interest in licensing PPL-103 for pain after it has entered human clinical trials. PPL is currently advancing both compounds through preclinical studies and then to proof of concept (Phase IIa) in humans. At or before that point is reached (in approximately three years) the company plans to license PPL-103 to one or more of those companies for acute pain and also license PPL-138 for chronic pain.
The following are examples of disclosed terms[3] for previous license deals for various drugs for pain including Licensor/Licensee; Stage; Upfront Fees; Milestone Payments; and Royalties as a % of Net Revenues (sometimes not disclosed):
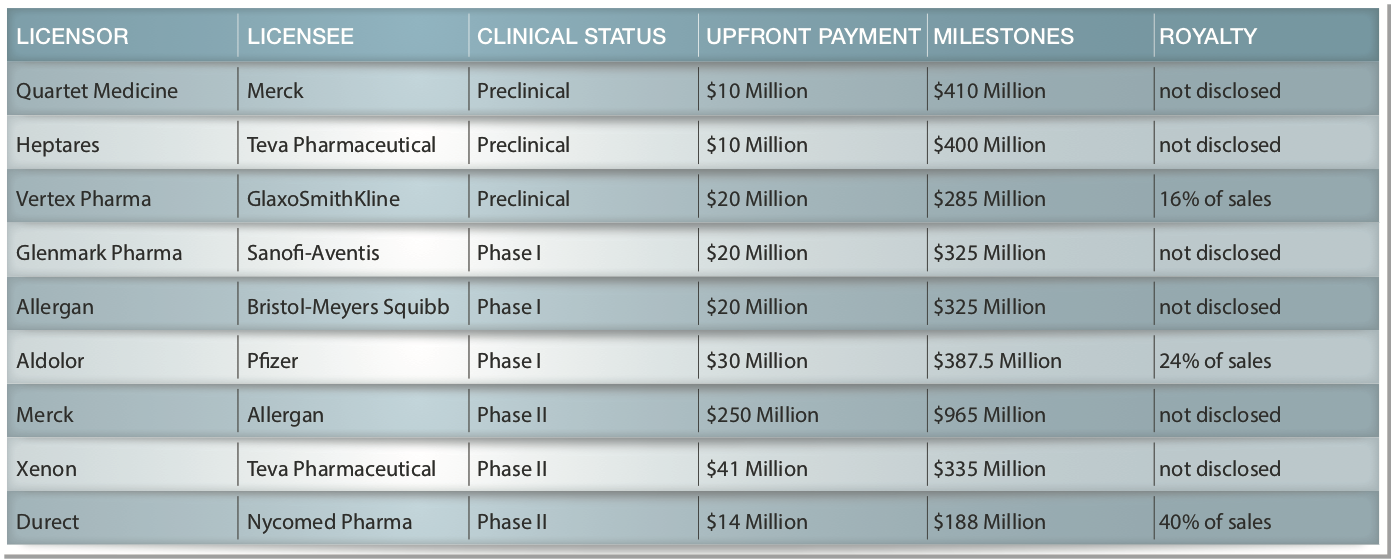
There is no assurance that PPL will enter into a similar license agreement. However, if these two compounds perform in humans similar to the way they have in animals we believe that their license potential would be very significant.
Progress
Unlike a pharmaceutical company, our “customer” is not the consumer. Rather, we seek to license our drugs to one or more leading pharmaceutical companies who will market them to consumers.
Licensing relies on strong patent protection. A Composition of matter patent (Patent No. 8,987,293) for PPL-103 was issued in the US in March, 2015 and PPL-103 has since received patent approval in major countries around the world. This patent also covers Methods of synthesis as well as Use. A Composition of matter patent (Patent No. 9,969,746) for all 25 compounds licensed from the University of Bath including PPL-138 was issued in the US in May, 2018 and has since received patent approval in major countries in the EU. Phoenix acquired exclusive rights to the University’s patents in July, 2020.
In addition to obvious potential partners in the pharmaceutical/pain/addiction space, Phoenix also plans to target the animal health market. PPL’s compounds are likely to be either unscheduled or scheduled at a low enough level for pet owners to be permitted to administer them to their pets at home. Many pets are currently in moderate to severe pain and their owners cannot get access to safe, potent pain medications that provide relief.
Our strategy for out-licensing is to advance PPL-103 and PPL-138 as quickly as possible into human clinical trials, through Phase I and into Phase II to Proof of Concept (POC) in humans at which point the drugs will be ideally positioned for out-licensing. It is possible, however, that we could enter into one or more license agreements or an acquisition or an IPO before that point is reached. At any one of those points investors could realize a return on their investment, although there is no assurance that any of those exit points will be achieved.
Success


The company was awarded a $2.7 million grant from the US Army Medical Research and Material Command (USAMRMC), which is currently funding the advancement of PPL-103 into human clinical trials. The company has strong support from scientists at the USAMRMC as well as the NIH / National Institute on Drug Abuse (NIDA).
The company also received a $185,000 grant from the NIH / NIDA (National Institute for Drug Abuse) to study PPL-103 for potential use as a cocaine addiction medication. It has already been demonstrated that the drug is effective at blocking relapse from cocaine addiction in rodents. Currently there are no medications that are effective for treating cocaine addiction.
On March 30, 2019, Phoenix closed its first Netcapital round which, at $1.1 million, was oversubscribed under SEC Rule CF.
The company is currently in the process of applying for new grants to support the advancement of PPL-138 into human clinical trials.
Press
- US Department of Defense grants funding for development of non-addictive opioid
- How to fight drug addiction: the shift to prescription drugs
- Phoenix PharmaLabs Exec Describes Novel Non-Addictive Opioid
- Research Declares Phoenix PharmaLabs' Opioid PPL-103 Safe
- Additionally, the news media is beginning to learn more about PPL-103. Here is a link to a story last November in STAT
- One Stepfather’s Quest: The Search For An Opioid That Won’t Lead To Addiction
- In Vitro and In Vivo Profile of PPL-101 and PPL-103: Mixed Opioid Partial Agonist Analgesics with Low Abuse Potential
- BioSpace: Phoenix PharmaLabs Announces Investment Offering to Support Additional Research of Non-Addictive Opioid
- Global Banking And Finance Review: Phoenix PharmaLabs Announces Investment Offering to Support Additional Research of Non-Addictive Opioid
KPIs
- Budget
- Schedules for manufacturing scale-up, preclinical and later clinical studies, and FDA regulatory requirements
- Specific manufacturing objectives and related metrics such as cost and lead time for sourcing raw materials, process time, yields, and end-product analytics such as purity and stability. Recently improvements in these metrics have resulted in identified savings of more than $600,000 in projected manufacturing costs next year.
- Successful results of animal studies and human clinical trials. Such studies that have been conducted so far have demonstrated the very promising characteristics of PPL-103 and PPL-138 described above as well as a number of related attributes.
- The detailed results of these studies can be seen in the PPL-103 Technical Narrative.
Team
- Motivation & Determination - Members of the management team have witnessed firsthand the devastating effects of opioid addiction. The CEO’s stepson has been seriously addicted to opioids for more than 18 years, and other members of the team have friends and family who were addicted and even died from opioid overdoses. The team strongly believes that PPL-103 and PPL-138 can play an important role in addressing the opioid crisis and is determined to get these drugs to market as quickly as possible.
- Scientific Knowledge and Creativity - The company’s scientists are highly regarded in their fields. They developed extensive expertise in medicinal chemistry and neuropharmacology at institutions such as Stanford Research Institute (SRI) and Torrey Pines Institute for Molecular Studies. Prominent scientists at leading institutions such as the National Institute on Drug Abuse (NIDA), Virginia Commonwealth University (VCU), the University of Michigan, and the Rockefeller University have also contributed to the company’s drug development technology.
- Lean Business Practices - The team is experienced in lean business practices. The company has a track record of accomplishing a great deal on a lean budget so far and intends to continue that discipline in the future.
Bill Crossman is a senior management professional with domestic and international experience as CEO, CFO and other senior management positions in enterprises ranging from entrepreneurial start-ups to Fortune 100 level companies. Mr. Crossman has a proven track record of successfully commercializing emerging technologies. He has assisted numerous early-stage companies to refine business strategies, commercialize new products, raise capital, license technologies, scale revenues and production, and expand into global markets. Crossman also has expertise in implementing lean business practices and continuous improvement strategies. Bill holds a BS degree from the U.S. Merchant Marine Academy at Kings Point and a MBA from the Haas School of Business at the University of California – Berkeley.
Dr. Lawson is an expert in medicinal and synthetic organic chemistry. As a senior Medicinal Chemist and Project Manager at Stanford Research Institute (SRI International) for 20 years, he headed the Neurochemistry R & D Group with responsibilities for the discovery and development of new compounds in neuroscience including analgesics, anti-convulsants, anxiolytics, and stroke therapeutics. While at SRI, Lawson collaborated for ten years with Dr. Toll, Chief Neuropharmacologist and Board Member of Phoenix PharmaLabs (see below), on analgesic drugs under NIH grant funding. During this period, Dr. Lawson discovered the initial class of opioids capable of relieving pain without the typical side-effect problems of morphine-like opioids. After leaving SRI, Lawson founded Phoenix PharmaLabs, Inc. for the purpose of renewing the development of non-addicting opioid compounds. John holds a BS degree with Phi Kappa Phi honors from Iowa State University and a Ph.D. from the University of Oregon. He was also a Postdoc. fellow at Syntex Corp.
Dr. Toll is currently Professor, Department of Biomedical Sciences, Charles E. Schmidt College of Medicine, Florida Atlantic University. He is also President of the International Narcotics Research Conference. Formerly he was Director of Neuropharmacology, Torrey Pines Institute for Molecular Studies, and prior to that he was Director of the Neuropharmacology Department at SRI International where he conducted basic and translational research in the fields of pain and addiction. An author of more than 130 peer-reviewed scientific papers, he is a recognized expert and leading researcher in the field of neuroscience, particularly in relation to addiction neurobiology and the pharmacology of drugs with potential addiction liability, such as opiates. Dr. Toll is a co-discoverer of the “nociceptin” opioid peptide and was part of the Opiate Research team at SRI that first researched potential non-addicting opioids. Dr. Toll has performed numerous selected assessments of neurochemical drugs for the National Institute on Drug Abuse (NIDA). As part of NIDA’s Opiate and Cocaine Treatment Discovery Programs he tested a very large number of compounds for affinities and activities at the three opioid receptors, five dopamine receptors, several 5-HT receptors, PCP receptors and sigma receptors. In conjunction with this project, Dr. Toll and his team published the definitive in vitro profile of opioid- and cocaine-related ligands. His lab has collaborated with many medicinal chemists to characterize the activity of a large number of compounds, including PPL’s compounds. Larry holds a BA degree in chemistry from the University of California, San Diego and a Ph.D. in biological chemistry from the University of California, Los Angeles as well as a Postdoctorate in biological chemistry at the University of California, Los Angeles and a Postdoctorate in pharmacology at Johns Hopkins University.
Mr. Chou is a serial entrepreneur and founding partner of Spectra Consulting Group where for over 25 years he has performed consulting on growth issues in emerging business development, including specialized consulting in strategic planning, capital acquisition, capital structures, organization re-engineering, process development, and dispute mediation. He specializes in start-up consulting for technology, life science, and consumer product companies. Mr. Chou’s corporate experience includes serving as a Chief Financial Officer, Controller, and as a CEO. He currently serves as an officer of several operating companies and sits on various boards as a director. He has participated in architecting numerous capital structures and has developed strategies for development-stage enterprises that have produced significant debt and/or equity investment. Mr. Chou’s clients are located across the western United States.
Mr. Tew brings over 25 years of professional bioscience sales, marketing and business development leadership experience to the company as a sales, marketing and development executive with American Hospital Supply, CooperVision, and Alcon. Throughout his career Mr. Tew championed many successful sales and product initiatives. In addition, Chris co-founded HealthWare Management Company, a healthcare software company, which was sold at a profit to Global Software. Chris holds a BA degree in mass communications from Brigham Young University.
Advisors and Investors
Dr. Levy is an experienced organic/medicinal chemist having contributed to the design of novel therapeutic agents targeting cardiovascular, cancer, inflammatory and CNS disorders. In his almost 30 years in the industry, Dr. Levy led interdisciplinary teams focused on kinase inhibitors, GPCR antagonists, matrix metalloproteinase inhibitors and cell adhesion molecules. His work is documented in over 30 peer-reviewed publications and over 11 issued/published United States patents. Dr. Levy is Founder and Principal Consultant of DEL BioPharma LLC, through which, he provides CMC services covering medicinal chemistry, API manufacturing and formulation development. Most recently Dr. Levy was the Vice President of Manufacturing at Censa Pharmaceuticals and the Director of Synthetic Chemistry at Intradigm Corporation. He is author and editor of three books covering aspects of mechanistic organic chemistry and carbohydrate chemistry. Dr. Levy received his academic degrees from the University of California – Berkeley (B.S., 1987) and the Massachusetts Institute of Technology (Ph.D., 1992).
Dr. Bradshaw is Executive Director, Center of Global Drug Development, PRA Health Sciences. He earned his doctoral degree from the University of South Carolina in 1989 and continued to complete two postdoctoral fellowships - the first in pharmacology and toxicology, at the Medical University of South Carolina, followed by one in drug metabolism, at Glaxo Research Institute. He joined GSK full-time as a senior scientist in the Biopharmaceutical Product Development group, serving as a core team member for numerous priority R&D programs. After leaving GSK in 2003, Tim held leadership positions of increasing responsibility in several biotech companies. In 2017, Tim joined PRA Health Sciences where he has continued to leverage his drug development expertise in providing strategic guidance to both established pharma and emerging biotech companies. Tim’s professional accomplishments have included demonstrated success in critical roles across the entirety of drug development / product lifecycle spectrum, guiding projects from lead compound identification through post-approval clinical trials.
Dr. Kreek is Senior Attending Physician and Patrick E. and Beatrice M. Haggerty Professor, Head of Laboratory of Biology of Addictive Diseases, The Rockefeller University. She is the recipient of numerous professional awards including: Betty Ford Award, Association for Medical Education and Research in Substance Abuse, 1996; Specific Recognition Award, Research in Science of Addiction, Executive Office of the President – Office of National Drug Control Policy 1998; R. Brinkley Smithers Distinguished Scientist Award, American Society of Addiction Medicine, 1999; Nathan B. Eddy Memorial Award for Lifetime Excellence in Drug Abuse Research, College on Problems of Drug Dependence, 1999; Fellow, New York Academy of Sciences, 2000; Columbia University College of Physicians and Surgeons Alumni Association – Gold Medal for Distinguished Achievements in Academic Medicine, 2004; Marian W. Fischman Memorial Lectureship Award, College on Problems of Drug Dependence, 2005; President, International Narcotics Research Conference 2002-2006. Dr Kreek holds a BA in Chemistry from Wellesley College where she was selected as a member of Sigma Xi and Phi Beta Kappa and where her thesis work allowed her to be named a Durant Scholar; she received her MD from Columbia College of Physicians and Surgeons.
Dr. Deshpande is founder and President of Universal Regulatory Inc, a regulatory affairs consulting firm focused on regulatory strategy, operations, compliance and medical writing. Over the years she has held industry positions of increasing responsibility in pharmaceutical development and regulatory affairs including Sr/Vice President/Head of Regulatory Affairs for publicly traded companies and small to mid-size biotechnology firms. As a consultant Dr. Deshpande has served as an advisor to more than 75 biotechnology/pharma companies, investment firms, Boards of Directors, providing strategic and tactical guidance on product development and regulatory affairs. Her background includes multi-functional expertise in Regulatory Affairs and pharmaceutical development with knowledge of domestic and international regulatory environments. She has provided regulatory guidance and strategic leadership for in excess of 100 Investigational New Drug (IND) including first-in-human products, global clinical trial applications, several successful marketing authorizations (US, EU), and negotiations with global health authorities. Deepa received her Masters and Ph.D. degrees in Pharmaceutical Sciences from West Virginia University and is RAC certified.
Jim is a board-certified toxicologist and senior consultant with over 40 years of experience in regulatory nonclinical safety evaluation of pharmaceuticals and chemicals with a focus in pharmaceutical industry for the past 30 years. He received his biology degree (BS) from Alma College and immediately started his career as a toxicologist with increasing responsibility over the years to include project management of nonclinical development for global programs with keen knowledge of the ICH and FDA guidelines, extending to drug development and life cycle management of drug products. Jim is currently providing consulting services to formulate and prepare nonclinical development plans and assist in study protocol design, data analysis and reporting. In addition, he assists with toxicology issue and problem solving as well as in interactions with and product submissions to regulatory agencies. Jim is a Diplomat of the American Board of Toxicology (DABT) and a member of the Society of Toxicology, American College of Toxicology and American Association for Laboratory Animal Science. In addition, he has authored/published several related publications.
Dr. Ko is Professor, Department of Physiology & Pharmacology, Wake Forest University School of Medicine. He has a 23-year history of research using rodents and nonhuman primates with a record of successful and productive research projects in areas of opioids and substance abuse. As PI or co-investigator on many university-, industry-, and NIH-funded grants, Professor Ko has studied approximately 60 newly developed experimental compounds and laid the groundwork for the proposed research by developing various behavioral and physiological measurements in rodents and monkeys following systemic and intrathecal administration of drugs. Dr. Ko has authored dozens of peer-reviewed scientific papers and is the recipient of several professional awards including: CPDD Early Career Investigator Award, 2002; Wyeth-Ayerst Young Psychopharmacologist Award, 2002 and Research Excellence Award, Wake Forest University School of Medicine, 2014-2015. Dr. Ko holds a Ph.D. in biopsychology as well as a Postdoctorate in anesthesiology/pharmacology from the University of Michigan.
Footnotes
- O’Connor EC, Chapman K, Butler P, Mead NM. (2011) The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 35:912-938
- Opioids Market Size, Share & Trends Analysis Report 2018 - 2025 by Research and Markets
- Selected license agreements with disclosed data since 2005 from Cortellis Deals Intelligence / Thomson Reuters Life Sciences (formerly Deloitte Recap) database
Use of Proceeds
If the offering's maximum amount of $1,070,000 is raised:
| Use | Value | % of Proceeds |
|---|---|---|
| Developing oral formulations | $400,000 | 37.4% |
| Preparation for Phase 1 Trials | $450,000 | 42.1% |
| Salaries | $167,570 | 15.7% |
| Intermediary fees | $52,430 | 4.9% |
Terms
This number includes all funds raised by the Company in this round on Netcapital. This is an offering of Common Stock, under registration exemption 4(a)(6), in Phoenix PharmaLabs Inc. This offering must reach its target of at least $10,000 by its offering deadline of June 18, 2021 at 12:59am ET. If this offering does not reach its target by the offering deadline, then your money will be refunded.
If the offering is successful at raising the maximum amount, then the company’s implied valuation after the offering (sometimes called its post-money valuation) will be:
Pitch Deck
Financials
These financial statements have been audited by an independent Certified Public Accountant.
SEC Filings
The Offering Statement is a formal description of the company and this transaction. It’s filed with the SEC to comply with the requirements of exemption 4(a)(6) of the Securities Act of 1933.
We’re also required to share links to each of the SEC filings related to this offering with investors.
Understand the Risks
Be sure to understand the risks of this type of investment. No regulatory body (not the SEC, not any state regulator) has passed upon the merits of or given its approval to the securities, the terms of the offering, or the accuracy or completeness of any offering materials or information posted herein. That’s typical for Regulation CF offerings like this one.
Neither Netcapital nor any of its directors, officers, employees, representatives, affiliates, or agents shall have any liability whatsoever arising from any error or incompleteness of fact or opinion in, or lack of care in the preparation or publication of, the materials and communication herein or the terms or valuation of any securities offering.
The information contained herein includes forward-looking statements. These statements relate to future events or to future financial performance, and involve known and unknown risks, uncertainties, and other factors, that may cause actual results to be materially different from any future results, levels of activity, performance, or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties, and other factors, which are, in some cases, beyond the company’s control and which could, and likely will, materially affect actual results, levels of activity, performance, or achievements. Any forward-looking statement reflects the current views with respect to future events and is subject to these and other risks, uncertainties, and assumptions relating to operations, results of operations, growth strategy, and liquidity. No obligation exists to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
More Info
Updates
- Jul 9, 2024New Phoenix update- you are not on the...
- Jul 12, 2023Phoenix PharmaLabs update: 1. PPL-138 continues...
- Oct 6, 2022Phoenix awarded 8.7m grant from NIDA to study...
- Sep 13, 2021Dr. Lawrence Toll, Chief Neuropharmacologist at...
- Jun 18, 2021Primary offering finalized, selling shares
- Jun 13, 2021NOTICE: The Phoenix PharmaLabs offering will...
- Jun 11, 2021Phoenix PharmaLabs Update: Phoenix recently...
- May 14, 2021Phoenix PharmaLabs Update: At the urging of...
- Jan 29, 2021Thought you would like to see some of the...
- Mar 30, 2019Primary offering finalized, selling shares
Ask a Question
Proofread your comment before submitting: once it's posted, you can’t edit or delete it. Investors are advised to review our Discussion Board Policy before submitting a comment. For the fastest help with the web site, email help@netcapital.com instead of commenting.
